Spatio-Temporal Logic of Adult Neurogenesis - Z. Chaker
Background
Research over the last three decades has demonstrated that new neurons can be generated in the adult brain, and integrate into pre-existing complex circuits. The process of adult neurogenesis is evolutionary conserved among vertebrates, including fishes, frogs, reptiles, birds, rodents and primates. It is sustained by a small population of undifferentiated cells, called neural stem cells (NSCs), which persist as embryonic vestiges in adult brains. These cells reside in tightly controlled micro-environments or niches, and co-exist in activated or quiescent states. The addition of young cells constitutes an important layer of adult brain plasticity, further enhancing its ability to adapt to diverse life experiences. However, the physiological relevance of adult-born neurons as well as the regenerative power of NSCs after injury are still highly debated to date, especially in mammals.
Research vision
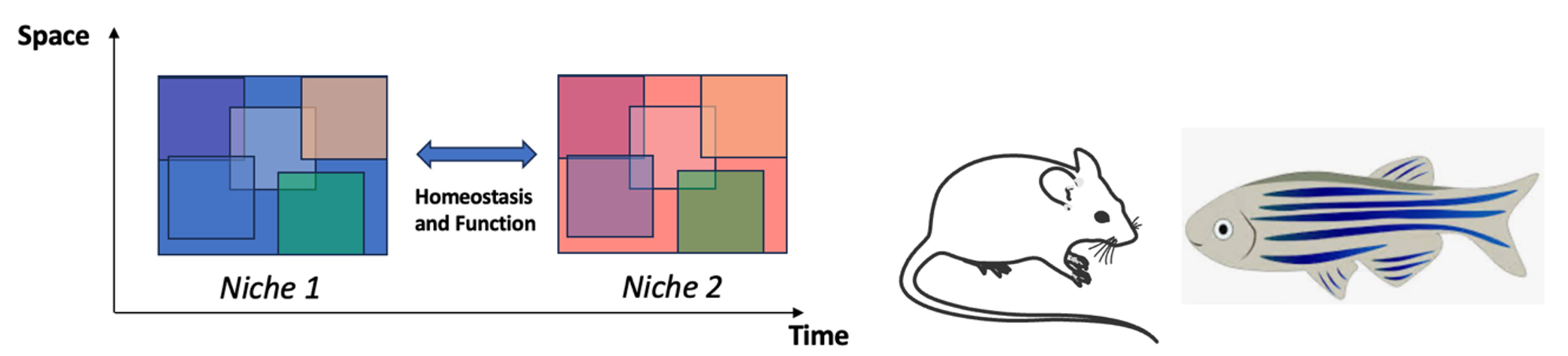
In the lab, we are investigating the cellular and molecular mechanisms allowing regionally-distinct pools of NSCs to coordinate their behavior in space (across niches) and time (from embryo to different phases in adulthood), under specific physiological and pathological conditions. Our work aims to better understand and eventually harness adult NSC potential, shedding new light onto the functional importance of adult neurogenesis for adaptive brain remodeling. We are following a developmental, comparative and circuit research perspective, using mouse and Zebrafish as two contrasting, yet complementary, model systems.
Main research interests
1- Build a brain Niche:StemCell interactome at unprecedented resolution
In the mouse brain, adult neurogenesis occurs in two main regions, the ventricular-subventricular zone lining the lateral ventricles (V-SVZ) and the dentate gyrus in the hippocampus (DG). There is a high regional diversity of NSC units or sub-populations in the (V-SVZ) and possibly in the dorsal versus ventral DG of the adult mouse brain. However, whether stem cells located in distinct domains are intrinsically different, or whether their regulation is mainly extrinsic with the niches themselves being regionalized, is still poorly understood.
2- Dissect the developmental origins of adult NSC heterogeneity
In mice, NSC regional identity is inherited from embryonic times and faithfully preserved in the adult. V-SVZ developmental and adult neurogenesis are not a continuous process. Instead, NSCs are set aside in a dormant state early in the embryo until needed in adulthood. In contrast, DG embryonic and adult NSCs derive from a common ancestor cell, supporting a model of continuous neurogenesis throughout development. Importantly, the molecular mechanisms underlying the transition from embryonic to adult stem cell identities across both niches are still unclear.
3- Reveal a circuit-level coordination of adult NSC behavior
Recently, it was demonstrated that quiescent NSC pools in the adult V-SVZ are selectively recruited in response to physiological state, under control of targeted long-range neuronal innervation. Similarly, DG neurogenesis was shown to be directly responsive to distant hypothalamic projections, which play pivotal roles in fine tuning cell replacement dynamics under pathological contexts. These data strongly suggest a specific circuit regulation of NSC behavior in both niches.
4- Comparative analysis to better understand transient neurogenesis in mammals
Besides constitutive neurogenesis, regionally-defined subpopulations of NSCs in the adult V-SVZ produce transient waves of functionally-relevant interneurons in response to physiological needs, such as pregnancy and motherhood. The question of whether specific NSCs are dedicated to generate behaviorally-relevant transient neurons, or whether they are the same as those sustaining continuous neurogenesis under homeostasis, is still totally unexplored. We hypothesize that transient neurogenesis in mammals is a powerful strategy favoring network plasticity and adaptability at the expense of growth and regeneration, and will test that by comparing Mouse and Zebrafish homologous neurogenic niches.
Methodologies
- Single cell RNAseq and Spatial Transcriptomics
- 3D lightsheet imaging
- CrispR-Cas9 genome editing tools
- Viral approaches for circuit mapping and manupulation (AAVs)
- Mouse and Zebrafish genetics for lineage tracing and intra-vital imaging
Team members
- Zayna Chaker (PI)
- Sabine Richard (CR)
- Suzy Markossian (IR)
- Violaine Hubert (IR)
- Rodriguez Renato (PhD)
- Priya Priya (PhD)
- Marco Uderzo (PhD)
- Gina Melgar (PhD)
Follow us
Our funding





