Thesis defense: Théodore Grenier
| When |
Sep 29, 2021
from 02:00 to 05:00 |
|---|---|
| Where | Amphitheatre SVT at ENS de Lyon |
| Contact Name | Théodore Grenier |
| Attendees |
Jury members: Rapporteurs : Pr. Irene Miguel-Aliaga et Pr. Jean-Marc Ghigo; Examinateurs : Dr. Samir Merabet et Dr. Nathalie Rolhion; Directeur de thèse : Dr. François Leulier. |
| Add event to calendar |
|

On september 29th,Théodore Grenier of the team of François Leulier will support his thesis entitled:
"Drosophila-Lactobacillus plantarum as a model of facultative nutritional mutualism"
Abstract:
Nutrition is the major environmental factor that determines juvenile growth: inadequate access to nutrients leads to delays in post-embryonic development. The gut microbiota is a key factor in this link between nutrition and growth: some symbiotic bacteria improve the growth of their host facing undernutrition. In our laboratory, we aim to understand the mechanisms of growth promotion by symbiotic bacteria. We use the larva of Drosophila melanogaster as a model. We can control its nutrition using a synthetic nutritional medium. Moreover, we can control its microbiota using axenic larvae (i.e. larvae that are free of any microbe) that we associate with growth-promoting symbiotic bacteria, Acetobacter pomorum and Lactobacillus plantarum (recently reclassified as Lactiplantibacillus plantarum). First of all, we tested how these bacteria influence the nutritional requirements of their host. We systematically removed a single nutrient composing the synthetic medium at a time to identify which nutrients are necessary to the growth of axenic larvae. We then showed that A. pomorum and L. plantarum can provide certain of these nutrients to their host, allowing it to develop on a diet deprived of these nutrients.
Moreover, we discovered an additional mechanism: although L. plantarum cannot produce the amino acid Valine, it promotes growth when its host faces amino acid unbalance due to Valine limitation.
To dissect this mechanism, we performed a genetic screen using a library of loss-of-functions mutants of L. plantarum. This approach allowed us to identify genes in the bacteria that are essential to this mechanism of growth promotion. We focused on one family of such genes, which encode ribosomal RNA and transfer RNA. L. plantarum mutants for these genes display a reduced capacity to promote its host’s growth. We then sought to understand how bacterial RNA may influence the growth of the host. GCN2 is a kinase conserved in all Eukaryotes that allow adaptation to amino acid unbalance. It is activated by the presence of rRNAs and uncharged tRNAs. Knocking-down GCN2 in the larva’s enterocytes using RNA interference inhibits the ability of L. plantarum to promote growth on a diet unbalanced in Valine. Moreover, we showed that association with L. plantarum activates GCN2 in the host’s enterocytes. Activation of GCN2 by L. plantarum depends partly on the genes encoding rRNAs and tRNAs in L. plantarum. Therefore, we propose that rRNAs and/or tRNAs produced by L. plantarum may activate GCN2 in the host’s gut, promoting adaptation of the host’s physiology to amino acid unbalance and growth. We analyzed the transcriptome of the host’s gut in different conditions and our results suggest that adaptation to an unbalanced diet may rely on the metabolism of the steroid hormone ecdysone.
Using complementary approaches of functional genetics in host and bacteria, we identified new mechanisms of host-microbes interactions that explain how symbiotic bacteria may promote the growth of their host in situation of undernutrition.
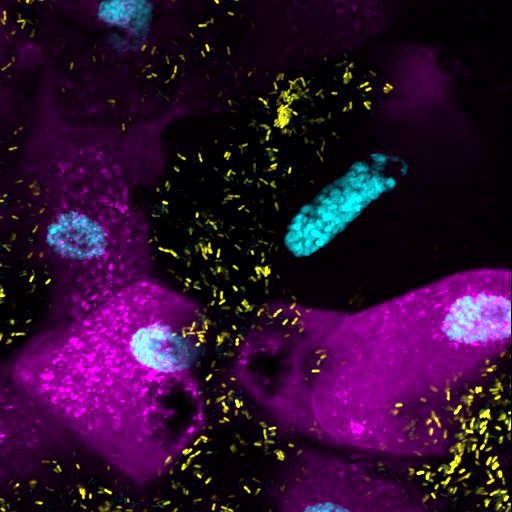
Confocal image of a Drosophila larva intestine. In cyan: cell nuclei, in magenta: a fluorescent reporter for GCN2 protein activity, in yellow: the bacterium Lactiplantibacillus plantarum.



