Thesis defense: Manuela Moreno
| When |
Sep 12, 2025
from 02:00 to 05:00 |
|---|---|
| Where | Salle D8.001 (site Buisson) |
| Contact Name | Manuela Moreno |
| Attendees |
Homaira NAWABI, Directrice de recherche, Université Grenoble Alpes / INSERM Rapporteure; Frank SCHNORRER, Directeur de recherche, Aix Marseille Université / CNRS Rapporteur; Jonathan ENRIQUEZ, Directeur de recherche, Ecole Normale Supérieure de Lyon / CNRS Examinateur; Bertrand MOLLEREAU, Professeur des universités, Ecole Normale Supérieure de Lyon Examinateur; Filipe PINTO-TEIXEIRA, Chargé de recherche - HDR, Université de Toulouse / CNRS Examinateur; Anne LAURENÇON, Chargée de recherche - HDR, Ecole Normale Supérieure de Lyon / CNRS Directrice de thèse. |
| Add event to calendar |
|
On September 12th, Manuela Moreno of the team of Jonathan Enriquez will support her thesis entitled:
"Role of DIP-α and Dpr10 in maintaining muscle innervation specificity in adult Drosophila leg"

Abstract:
Axon-muscle connectome, defined as the stereotyped and precise wiring of motor neuron (MN) axon terminals contacting each muscle, is essential for coordinated motor function. This connectivity is established during development through genetically encoded programs that guide MNs to their specific muscle targets. While these developmental mechanisms are well characterized, their contribution for the long-term maintenance of neuromuscular circuits in adulthood is poorly understood. Increasing evidence suggests that neuromuscular connections are not passively preserved but instead require continuous molecular support, as adult neuromuscular junctions (NMJs) undergo remodeling in response to activity, aging, and circadian cues. Notably, several molecular players involved in synaptic partner recognition during development, including cell adhesion proteins and axon guidance molecules, remain expressed in adult MNs and muscles. This persistent expression suggests that developmental molecular pathways may be crucial to sustain synaptic specificity and circuit integrity throughout life.
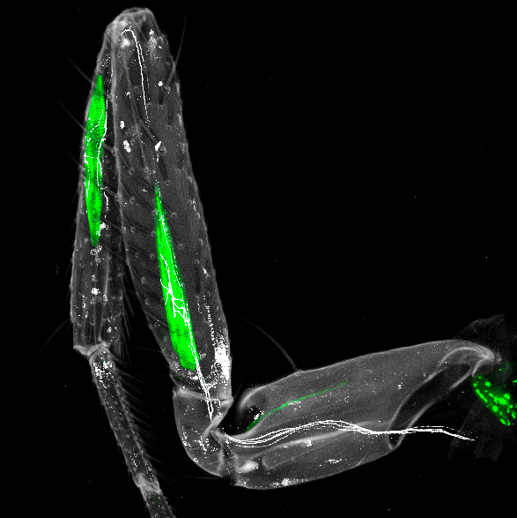
In this study, we investigate the role of the immunoglobulin superfamily member DIP-α, and Drosophila ortholog of human IgLONs, in maintaining neuromuscular connectivity in the adult leg. During development, DIP-α and its binding partner Dpr10 control the terminal targeting of a specific subset of three MNs to three different leg muscles in the adult.
Using inducible, adult-specific RNAi, we demonstrate that DIP-α expression is both necessary and sufficient to maintain specific neuromuscular connections in adult flies. Loss of DIP-α in mature MNs leads to defects in terminal innervation of DIP-α expressing MNs, such as the decrease of mean branch length and number of nodes compared to controls. Conversely, targeted re-expression of DIP-α in adult MNs of DIP-α null mutants is sufficient to restore correct terminal branching, even in aged flies, highlighting an unexpected degree of plasticity within adult neuromuscular circuits.
To further explore the muscle-specific environment that may contribute to synaptic specificity, we performed single-nucleus RNA sequencing (snRNA-seq) of the adult femur and tibia. This analysis uncovered distinct transcriptional signatures across the four femoral muscles, suggesting that synaptic maintenance mechanisms are muscle-specific and regionally patterned. Notably, several transcription factor and transmembrane proteins are differentially expressed between muscles, raising the need for further exploration of muscle specific axon-muscle proteo-interactome. Differential expression of transcription factors, transmembrane proteins, and other regulators suggests that each muscle-muscle fiber provides a unique molecular context that may shape MN targeting and maintenance.
Together, our findings identify DIP-α as a key regulator of specific MN targeting maintenance in the adult leg and support a model in which developmental molecular codes are expressed and repurposed in adulthood to actively maintain and preserve neuromuscular connectivity. These codes expressed in very specific subgroups of MNs and muscles are needed for an active maintenance process of unique connections in adult locomotor system. This work provides new molecular insight into how motor circuits are continuously built and maintain throughout the animal’s lifetime.


