Current projects
Development of astrocytes vs motoneurons
How do two types of cellular development, stochastic and stereotypic, coordinate to build a functional system?
The glial cells play many functions in the central nervous system (CNS) such as establishment of synapses or maintenance of neuronal activity. Astrocytes are a particular type of Glia that send processes deep into the neuropil, in close contact with synapses. Astrocytes are important for the function, homeostasis, development, and modulation of neuron synapses. Despites the importance of astrocytes in the CNS function and development, they remain poorly studied compared to neuron development. Strikingly, leg motoneurons and astrocytes use different modes of development even though they are derived from the same stem cells. Whereas motoneurons both display a stereotyped birth order and a TF code controlling their morphologies, the number and the individual morphologies of astrocytes born from these stem cells are highly plastic. The molecular basis of astrocyte plasticity is unknown. How do two types of cellular development, stochastic and stereotypic, coordinate to build a functional system?
Our Goal is to understand the developmental logic of astrocyte development by using powerful genetic toll such as tha MARCM, MARCMbow or the cisMARCM techniques.
Development of Motoneurons
How can a stem cell generate Motoneurons with different morphologies during development?


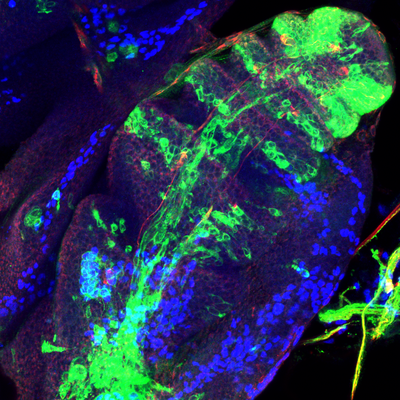
2016 Drosophila Image Award Finalist
The rhythmic and precise pattern of locomotion is linked to the coordinate contraction of muscles which are innervated by a complex wiring of axons controlling the timing and the intensity of muscle activity. The architecture of this precise cabling is built during the morphological differentiation of motoneurons. How can stem cells generate during development the huge diversity of motoneuron morphologies?
To discover new mechanism controlling the generation of neural diversity trough time we have chosen Lin A as a model system, a lineage producing 60% of all leg motoneurons for which we have powerful genetic tools to visualize its development from the neuroblast stage until the adult. From this objective we combine immunohistochemistry, smFISH and genetic techniques with computational approaches.
Development of muscle innervation
How do two tissues with a stereotypic program communicate during development to construct a functional system?
Motoneurons innervating fly leg or vertebrate limb muscles send axons out of the CNS during development to meet their muscle partner localized in the fly imaginal disc or the vertebrate limb. The wiring between an axon and its muscle partner is stereotyped between animals and under the control of transcriptional programs during development. The cellular mechanism and the effector genes of this transcriptional program, implicated in the stereotypic axon-muscle recognition in appendages, are unknown.
Advances in axon-muscle recognition have been made in the Drosophila larval neuromuscular system, where several secreted proteins or transmembrane proteins playing attractive and repulsive roles have been discovered, providing a conceptual basis of axon-muscle recognition. However, the locomotor system of the Drosophila larva has a singular architecture and development. Each larval body wall muscle is composed of a single fiber, innervated by axons after muscle differentiation. In contrast, mouse limb and Drosophila leg muscle are composed of several fibers, and muscle-axon recognition occurs early during development, before the formation of appendages. Data in Grasshopper leg even suggest that axons recognize muscles at the stage of muscle precursor cell (before the process of fusion). In Fly, the imaginal disc (immature leg) of wondering larvae is crossed by a single nerve). MN axons defasciculate from this nerve very early during leg metamorphosis, 20 hours before the process of fusion, suggesting that axons could recognize muscle precursor cells before the process of fusion. It is at this developmental stage that unique connections between developing muscle and immature MN will take place. How do two tissues with a stereotypic program communicate during development to construct a functional system?
For this objective we combine immunohistochemistry, genetic techniques with single cell profiling to discover the effector molecules controlling the unique recognition between an axon and a muscle fiber.
Maintenance of muscle innervation
Are the same gene networks necessary for the establishment and maintenance of muscle innervation?
Each motoneuron axon terminal innervates specific muscle fibers and displays an unique architecture defined by their shape and number of synaptic boutons The unique wiring and architecture of motoneuron ax
on terminals ensure the proper contraction of muscles allowing correct movements. In adult, the
maintenance of muscle innervation architecture is essential to keep the locomotor system functional. Motoneuron axon terminals are dynamic structures. Those innervating Drosophila flight muscles are dismantled and reestablished every day. During the night, the number of synaptic boutons decreases, while the correct number of boutons is restored during the day. Despite the dynamic nature of the motoneuron axon terminals, their unique wiring and architecture is always maintained. Peripheral nerves damaged by mechanical, chemical and biological stresses have the capacity to regenerate. For example, ablation of wing sensory neurons in Drosophila is followed by axon regeneration. In Crayfish, when a motor nerve is cut, it regenerates and innervates again the correct muscles. In humans, when a peripheral nerve is injured, axons regrow with a speed of 1 mm per day and muscle function can be recovered. Thus, the maintenance of muscle innervation across physiological conditions or after injury must preserve unique wiring and architecture of motoneuron axon terminals to sustain locomotor function.
To achieve our goal, we combine RNA profiling of motoneurons and muscles with state-of-the-art techniques and a newly designed genetic tool to visualize and modify the genotype of cells adult flies, and determine the impact of these manipulations on locomotion with unique behavioral technologies (Flywalker).
Technological axis
As part of the Spatial Cell ID initiative—a national consortium for spatial transcriptomics funded by the ANR, which we co-lead alongside Yad Ghavi-Helm and Teva Vernoux—we are developing a pipeline for spatial transcriptomics based on 3D MERFISH. Our broader objective is to integrate 3D spatial proteomics into this pipeline and, in collaboration with Yad Ghavi-Helm, to incorporate 3D spatial genomics as well.

For more information, visit the project's website.








