Revealing an unexpected contribution of intestinal ecdysone signaling to microbiota-dependent developmental plasticity
The Leulier team has just published a new article entitled:
“Ecdysone-mediated intestinal growth contributes to microbiota-driven developmental plasticity under malnutrition” in Development.
The article is accompanied by an interview and a Research Highlight.
Congratulations to the team for these great results!
Abstract:
Growth and maturation in animals must remain tightly coordinated throughout development, including under unfavorable nutritional conditions. In both insects and vertebrates, this coordination relies on systemic hormonal signals, but it is also influenced by the gut microbiota, whose precise role in developmental adaptation remains incompletely understood.
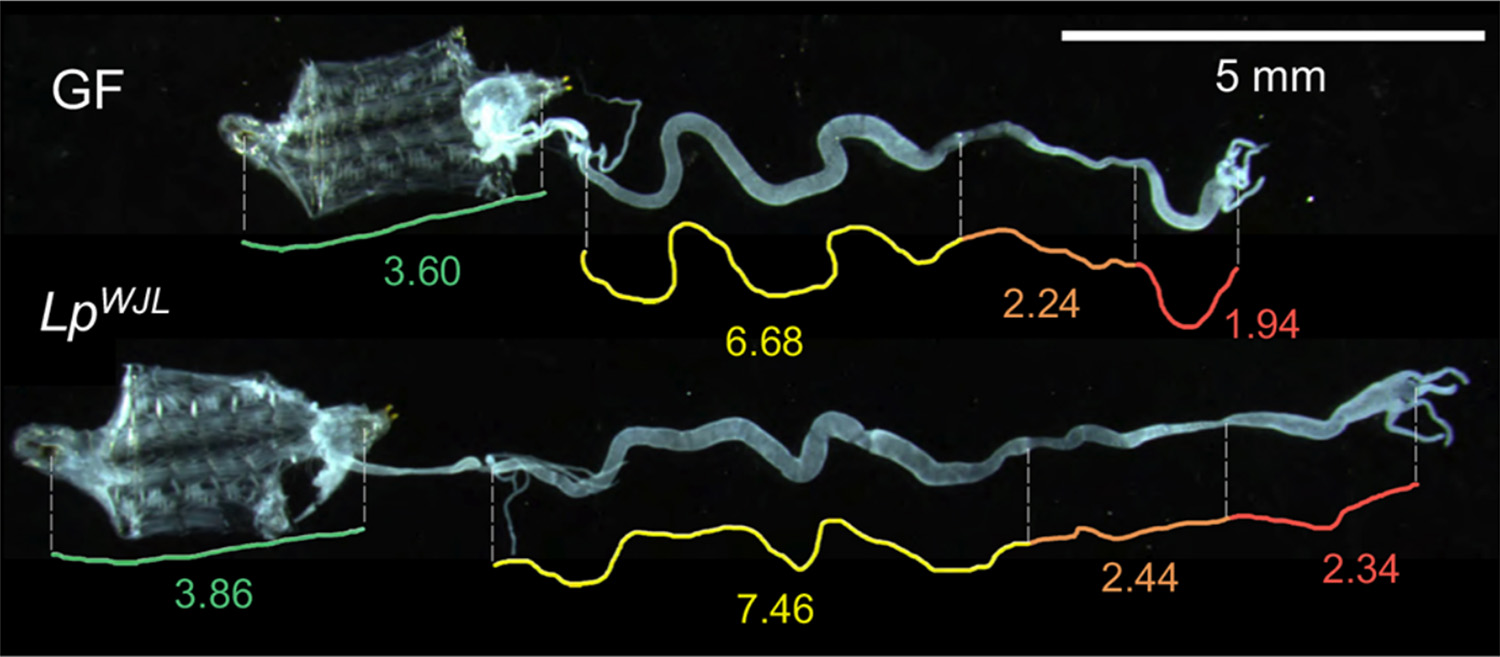
This study investigates the interactions between nutrition, microbiota, and hormonal regulation of development using Drosophila as an experimental model. The authors show that under chronic malnutrition, association with a commensal gut bacterium, Lactiplantibacillus plantarum, induces selective growth of the larval intestine without comparably affecting other organs. This adaptive intestinal growth contributes to maintaining coordinated systemic growth and maturation despite dietary restriction.
Using transcriptomic and functional analyses, the study identifies an unexpected local activation of ecdysone signaling—a key steroid hormone in insect development—within intestinal epithelial cells. The bacterium locally stimulates the conversion of inactive ecdysone into its active form, 20-hydroxyecdysone, via the enzyme Shade. Activation of the ecdysone receptor in the intestine is required for the beneficial effects of the microbiota on intestinal growth and final organismal size. Pharmacological activation of this hormonal pathway partially mimics the bacterial effect, demonstrating its functional sufficiency.
Together, these findings reveal a previously unrecognized role for intestinal steroid signaling in microbiota-driven developmental plasticity. They show that microbial signals can locally modulate a hormone classically viewed as systemic, thereby adjusting digestive capacity and overall growth to the nutritional environment. This work opens important perspectives on evolutionarily conserved mechanisms linking the gut microbiota, steroid metabolism, and developmental control, with potential implications for understanding microbiota–endocrine interactions in animals, including mammals.